Safety and Reliability
Approach and Policy
As a company involved in food and medicine, the Takara Group believes that it is important that customers find us reliable. In order to continue to be a corporate group trusted by customers into the future, we have established, and are working on, the Takara Group Quality Policy.
Takara Group Quality Policy
Based on our corporate philosophy, which is “Contributing to the creation of a vital society and a healthy lifestyle through our fermentation technology and biotechnology in a way that achieves harmony with nature,” we will bring safe and reliable products and services to customers throughout the world.
- 1.We will listen carefully to our customers and pursue quality that satisfies them.
- 2.We will work together as a group on quality assurance activities throughout the entire process until products are delivered to customers.
- 3.We will comply with the relevant laws, regulations and voluntary standards.
- 4.We will live up to the trust of our customers by making efforts to provide them with appropriate information that is easy to understand.
Targets
| Initiative themes | Specific measures | Targets |
|---|---|---|
| Ensuring safe and reliable quality at Takara Shuzo | Maintain safety management system (FSSC22000 at six plants in Japan) |
[ Takara Shuzo ] Maintain FSSC22000 at all six plants in Japan. (*Matsudo, Kusu, Fushimi, Shirakabegura, Kurokabegura, and Shimabara) |
|
Creating safe products at Takara Shuzo - Examine compliance with laws, regulations, and voluntary standards at the design stage - Stable procurement of raw materials whose safety has been confirmed - Maintain manufacturing lines that ensure safe and reliable quality |
[ Takara Shuzo ] - Ensure to check the safety quality in each of product design, raw material procurement, and production phases, thereby further enhancing the quality control system. - Maintain a completion rate of 100% for the auditing and quality control assessment of new outsourcing contractors and raw material suppliers. - Maintain a completion rate of 100% for checking the safety of raw materials with warranties. |
|
| Pursue customer satisfaction (ISO10002) |
[ Takara Shuzo ] Improve the process of handling complaints and increase the knowledge of departments handling them. a. The Quality Control Department provides training sessions at least once a year to improve the knowledge of Customer Contact Office staff members. b. Test the degree of understanding the manual on customer visits in order to improve the quality of responding to customers at the time of vising them. (Achieved a test completion rate of 100%) |
|
| Ensuring safe and reliable quality at Takara Bio Group | Maintain the quality management system (ISO9001, etc.) at Takara Bio Group |
[ Takara Bio ] - Maintain ISO certifications obtained by business offices and strive to increase quality and customer satisfaction. Expand the scope of areas to obtain ISO certifications where necessary. - Maintain licenses, registrations, and the like necessary for operating business. |
| Comply with and maintain various quality, manufacturing, and safety standards, including GMP/GCTP (*1), and third-party certification systems | ||
| Appropriately disclose product information |
[ Takara Bio ] Provide information on safety data sheet *2 (SDS) of Takara Bio products (reagents) in various languages (Japanese, English, Chinese) by FY2025. |
|
| Ensuring safe and reliable quality at Takara Shuzo International Group |
Efforts toward the establishment of a global quality assurance system - Understand information on overseas food safety laws and regulations and comply with them - Promote the obtainment of certifications on food safety by overseas group companies. |
[ Takara Shuzo International ] - Maintain a 100% rate of compliance with food laws and regulations in export destination countries. - Conduct quality audits on overseas group companies by FY2025, and take appropriate corrective measures if any quality risk factors are found. |
- *1 GMP:Good Manufacturing Practice, GCTP:Good Gene, Cellular, and Tissue based Products Manufacturing Practice
- *2 SDS:Safety Data Sheet (safety data sheets for chemical substances)
Related SDGs
Initiatives
Ensuring safe and reliable quality at Takara Shuzo
Maintain safety management system (FSSC22000 at six plants in Japan)
We are committed to maintaining the highest standards of food safety and quality across all operations. All six plants within Japan—Matsudo, Kusu, Fushimi, Shirakabegura, Kurokabegura, and Shimabara—are certified under FSSC 22000, an internationally recognized food safety management system. This certification demonstrates our dedication to risk management, global compliance, and consumer trust.
Creating safe products at Takara Shuzo
Examine compliance with laws, regulations, and voluntary standards at the design stage
Our design review process covers every aspect of product development—from raw materials and packaging to manufacturing processes, labeling, and shipping—ensuring quality, safety, and regulatory alignment. To strengthen expertise, we regularly conduct training sessions for staff on key regulations, including the Liquor Business Act, Food Labeling Act, and Act against Unjustifiable Premiums and Misleading Representations. This proactive approach ensures that our products meet the highest standards of safety, functionality, and transparency.
-
Stable procurement of raw materials whose safety has been confirmed
With regard to procuring raw materials, we strive to use only raw materials for which we have confirmed the entire procurement route without exception and that have warranties attesting to their quality, safety, and legality. In addition to these efforts, we conduct regular quality audits of suppliers that supply raw materials and carry out analysis of residual pesticides/herbicides, heavy metals, stable isotope ratios of alcohol imported from overseas, etc. according to the conditions of raw materials and suppliers, in order to improve the reliability of raw material quality by ensuring that any hazardous substances or foreign materials are not mixed in the raw materials.
-

Ingredient analyses and inspections using analytic instruments
Maintain and improve manufacturing lines that ensure safe and reliable quality
Manufacturing lines at out plants incorporate new technologies, and strict management of equipment maintenance and production processes ensures high quality. With inspection machines, we inspect all products for external appearance, foreign substances, and labeling/printing. Furthermore, in the inspection department, organoleptic inspections are carried out by expert panelists, and ingredient analysis and inspections are handled by state-of-the-art analytic instruments. Under the Medium-Term Management Plan for 2025, we are investing ¥14.3 billion in an effort to create safe and reliable products.
-
Efforts that were reinforced in response to the voluntary recall of canned chu-hi products
The voluntary recall of canned chu-hi product in May 2021 was caused by a lack of maintenance of product manufacturing facilities and inadequate inspection systems at Takara Shuzo plants. To prevent recurrence going forward, the Safety and Security Promotion Office was newly established in the fiscal 2022 as a company-wide safety and security management organization. We are striving to strengthen the promotion of quality control and quality improvement. As the first major initiative of recurrence prevention measures, we reidentified quality risks based on past cases and made necessary improvements. As the second initiative, we introduced automated inspection machines for all products. As the third initiative, we have added latest technologies and functions not only at the time of installation and renewal of facilities, but also at the time of regular inspections, thereby making continuous improvements.
-

Adopted inspection machines
-
Recognition as Outstanding Food Hygiene Facilities by the Minister of Health, Labour and Welfare
Takara Shuzo Co., Ltd.’s Fushimi Plant (West) and Shirakabegura have been honored as Outstanding Food Hygiene Facilities in the FY2025 “Commendation for Meritorious Contributors to Food Hygiene and Outstanding Food Hygiene Facilities,” jointly organized by Japan’s Ministry of Health, Labour and Welfare and the Japan Food Hygiene Association.
This commendation aims to set exemplary standards by recognizing facilities that demonstrate exceptional food hygiene practices.
Both plants have consistently maintained FSSC 22000 certification, ensuring the proper operation of food safety and quality management systems. Their robust production management and commitment to excellence were highly evaluated, leading to this prestigious recognition. -

Commendation Ceremony
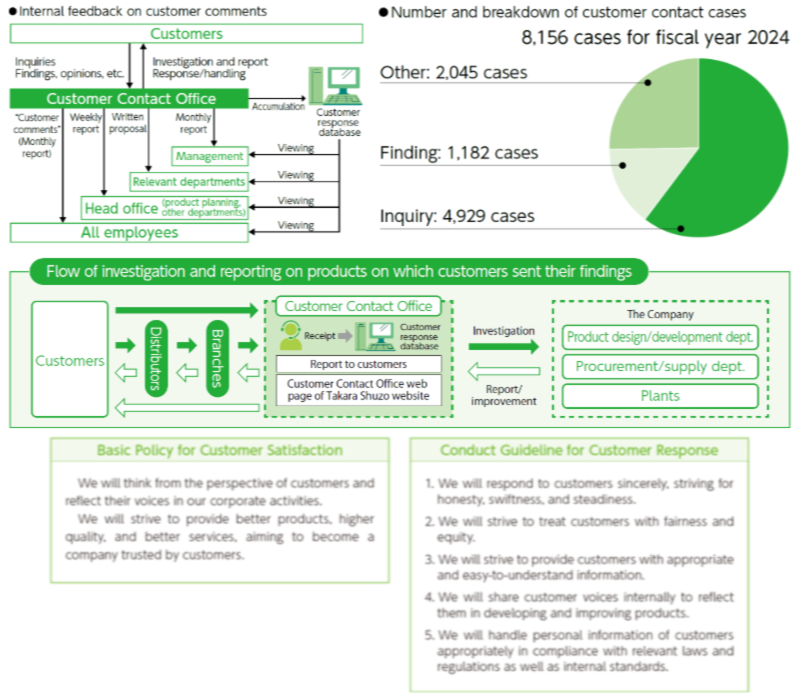
Pursue customer satisfaction (ISO10002)
In fiscal 2024, the Customer Contact Office received 8,156 comments. These include a wide variety of inquiries, feedback, and findings about products, and Takara Shuzo listens carefully to each of these comments, striving to deliver products and improve services in order to satisfy customers. In addition, videos on frequently asked questions are posted on the website of the Customer Contact Office, and efforts are being made to make them easier for customers to understand. We have made a declaration of conformity to ISO 10002 (targeting management systems for handling customer requests and complaints) and thus clarified our specific efforts and responsibilities to improve customer response, thereby continually Maintenance of the Food Safety Management striving to improve these processes.
Ensuring safe and reliable quality at Takara Shuzo International Group
Efforts toward the establishment of a global quality assurance system
Takara Shuzo International has newly appointed a person in charge of overseas quality assurance and quality control to further strengthen its quality assurance system. At meetings regularly held with the executives of our overseas group companies, we work to share our quality policies and exchange and collect information on food safety. In coordination with the Takara Holdings Group Quality Assurance Office, we conduct quality audits on our overseas group companies to identify quality risks and improve processes, thereby ensuring safer and more reliable quality.
Ensuring safe and reliable quality at Takara Bio Group
Maintain quality management system (ISO9001, etc.)
In the Takara Bio Group, Takara Bio Inc., Takara Biotechnology (Dalian) Co., Ltd., DSS Takara Bio India Pvt. Ltd., and Takara Bio Europe S.A.S have obtained the ISO 9001 certification for quality management systems. On top of this, Takara Bio USA, Inc. and Takara Biotechnology (Dalian) Co., Ltd. have obtained the ISO 13485 certification for diagnostic agent quality management systems and engage in strict quality control.
Comply with and maintain various quality, manufacturing, and safety standards, including GMP/GCTP, and third-party certification systems
At Takara Bio, the Center for Gene and Cell Processing, which is engaged in the CDMO business that supports the development and manufacturing of regenerative medical products and in the manufacturing of investigational products used in clinical trials of in-house gene therapy projects, operates its business in accordance with GMP/GCTP (Good Manufacturing Practices and Good Gene, Cellular, and Tissue-based Products Manufacturing Practice). We have also acquired certifications and permits such as registration as a clinical testing laboratory, designation as a foreign cell processor, and business license as a manufacturer of regenerative medical products. In the gene analysis and inspection business, we have been taking measures such as acquiring certifications from third-parties such as a recognition from the College of American Pathologists: Laboratory Accreditation Program (CAP-LAP).
Appropriate disclosure of product information
We actively publish materials related to product safety. We disclose and provide appropriate information in accordance with laws and regulations in various languages, including product manuals, Certificate of Analysis (CoA), SDS (Safety Data Sheet), the indication of poisonous or deleterious substances in accordance with the Poisonous and Deleterious Substances Control Act, and the labeling of products that fall under living modified organism (LMO) based on the Act on the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms (the Cartagena Act).


