Biomedical Business
Reagents and instruments, CDMO, and gene therapy
Business Activities
A global platformer providing infrastructure for the life science industry
Takara Bio Group supplies reagents and instruments and provide CDMO services
for cutting-edge regenerative medicine products to customers inside and outside Japan.
-
Reagents and Instruments
Takara Bio supports academic and corporate life sciences activities by offering reagents and instruments.
-
Contract Development and Manufacturing Organization (CDMO)

Through our CDMO business, Takara Bio provides contract services for biologics development and manufacturing, in all steps from process development to product manufacturing, for clients such as pharmaceutical companies.
-
Gene Therapy

Takara Bio is working on the commercialization of its proprietary platform technology for biologics development.
Takara Bio Group
Contributing to global gene and cell research with a broad product lineup and highly specialized technical support
Takara Bio’s reagents and instruments are favored by researchers in both Japan and abroad.
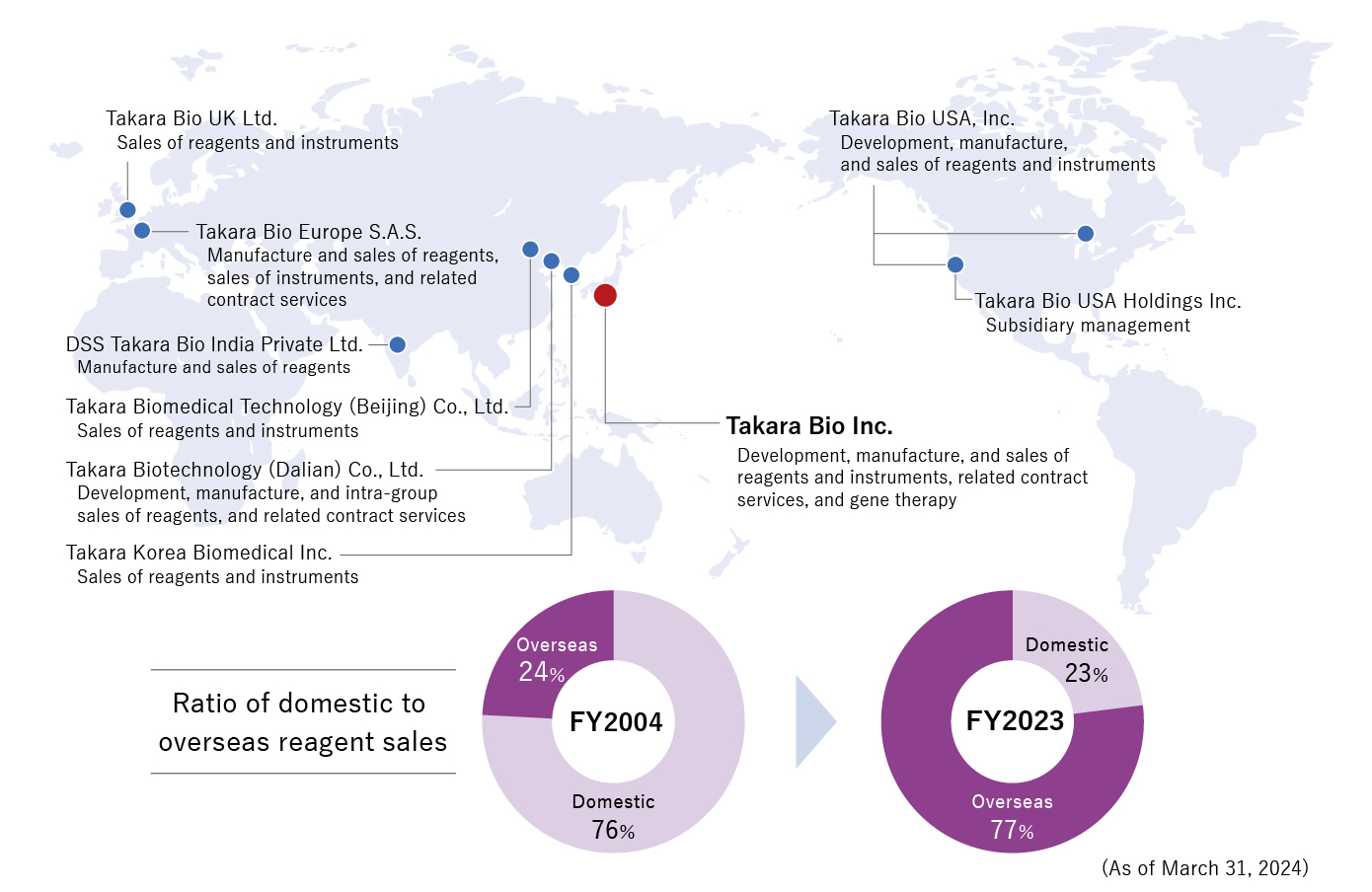
We develop and deliver products tailored to the needs of local researchers through our manufacturing centers in the U.S., Europe, China, and India, along with our global sales network, and provide fine-tuned support using our advanced technical capabilities. We particularly focus on life science research in the areas of genes and cells, and have built research infrastructure through our approximately 10,000 reagents and highly versatile instruments.
【Global expansion of our reagent and instrument business】
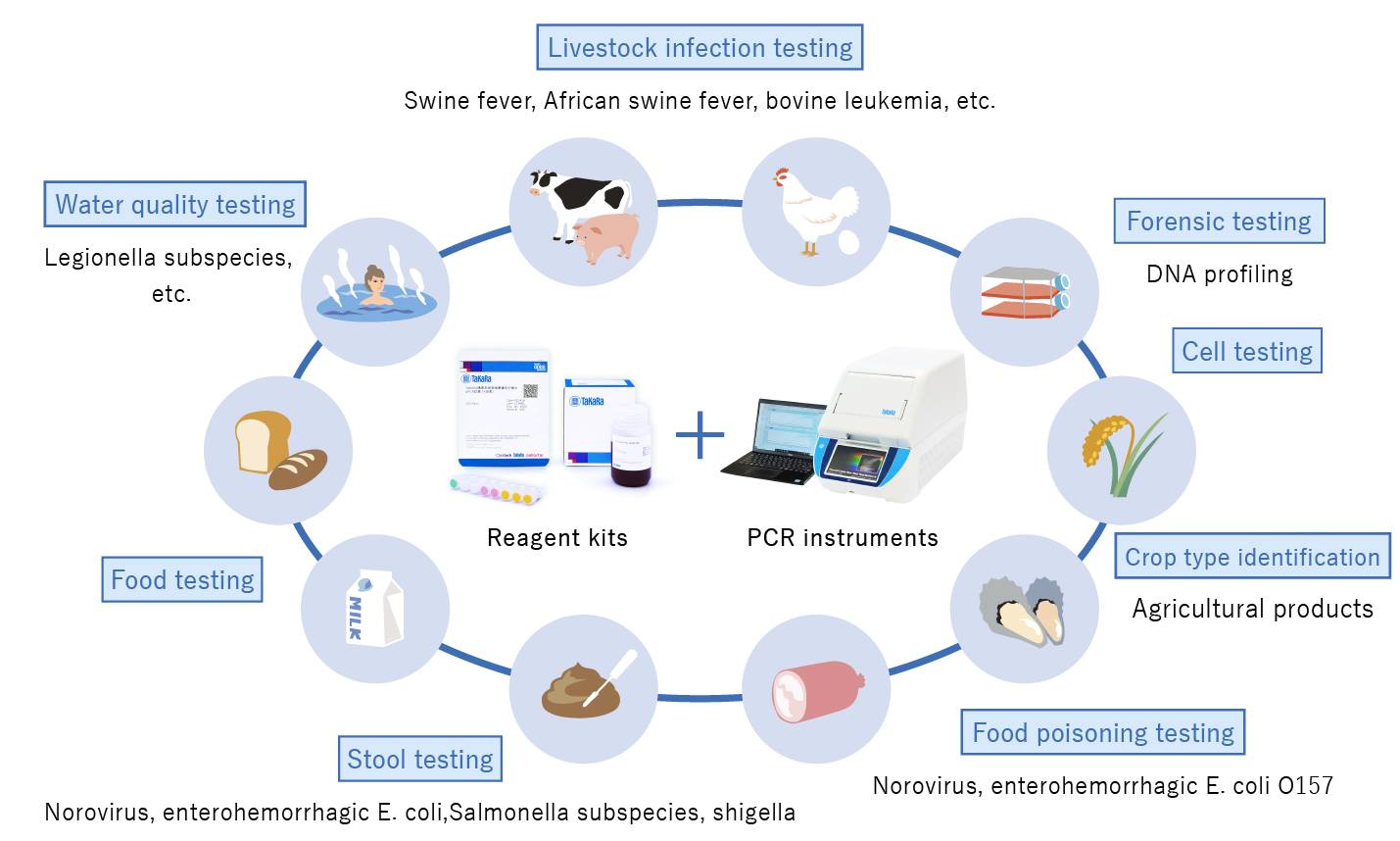
Promoting health and broadly supporting industry needs with PCR products
Takara Bio became the exclusive PCR system distributor in Japan in 1988, and began manufacturing PCR systems in-house in 1993.
We have sold over 30,000 PCR-related devices to date. PCR testing is used for a variety of purposes in areas such as basic research, infectious disease testing, and environmental testing, in settings that include not only research laboratories but also medical institutions and public health laboratories. When demand for PCR testing spiked due to the COVID-19 pandemic, we ensured a stable supply of PCR kits through a system that enabled us to manufacture enough PCR kits to conduct 12 million reactions per month.
【Expansion of PCR-related Business into Industrial Fields】
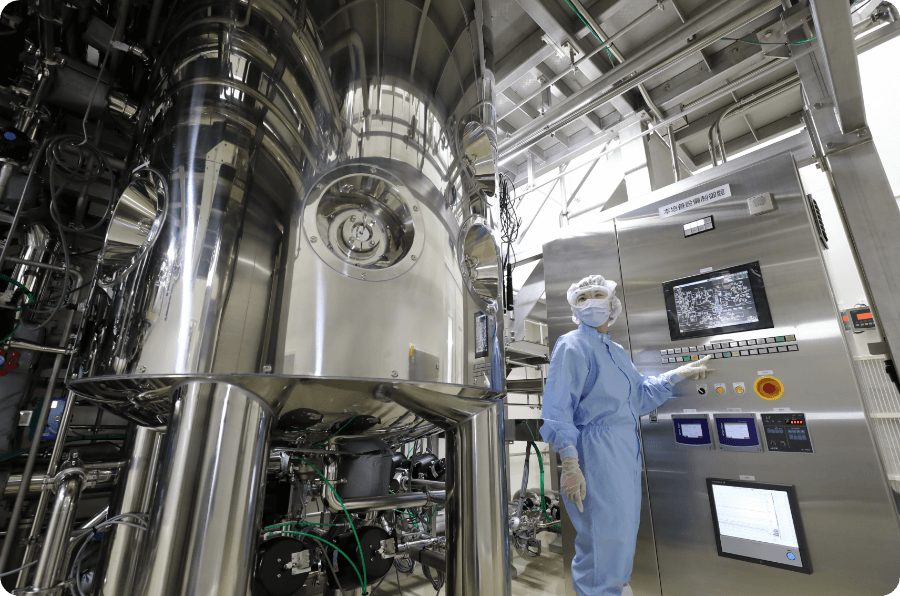
Leveraging technical expertise to support development and manufacturing of cutting-edge biopharmaceuticals and bring them to those who need them
Takara Bio has a long history of developing platform technologies for biologics, and in 2008 became the first company in Japan to conduct clinical trials of gene therapy products. We also entered the CDMO business earlier than other companies and have been operating and running a CDMO facility, ahead of our competitors. This facility, called the Center for Gene and Cell Processing (CGCP), is a large-scale one-stop center for development, manufacturing, and quality testing of gene therapy products. As the market for next-generation medicine expands, we will continue working to bring these treatments to those who need them.
Takara Bio contributes to the health of humankind through the development of revolutionary biotechnologies such as gene therapy.

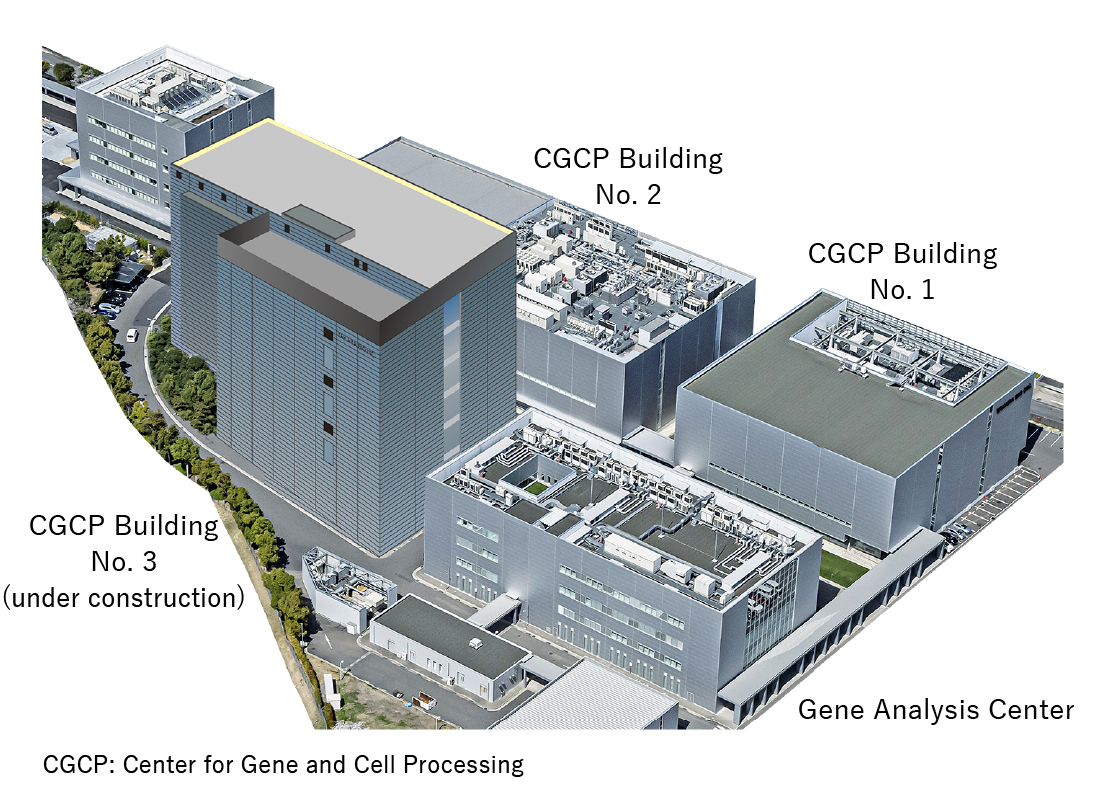
【Largest CDMO facility for cell and gene therapy products in Japan】
-
Construction plan for new CGCP Building No. 3
To be used for emergency vaccine production and routine CDMO services/drug discovery business
Building area: Approx. 2,650 m2
Total floor area: Approx. 16,400 m2
Structure: 7 stories above ground with seismic isolationConstruction started: April 2024
Construction to be completed: June 2027
Biomedical Business History
Takara Bio Group
Contact information for Takara Bio
-
Phone

+81 077-565-6920
Hours: 9:00 AM to 5:00 PM
(except on weekends and holidays) -
Email



Overseas Business
Overseas alcoholic beverages
and Japanese food wholesale